Accumulating evidence suggests that the aggregation of islet amyloid polypeptide (IAPP, a.k.a. amylin) is associated with β-cell death in type 2 diabetes (T2D). IAPP is co-secreted with insulin by pancreatic beta-cells, and also works together with insulin to control the serum glucose level. In vitro studies suggest that IAPP is one of the most amyloidogenic peptide, which forms amyloid fibrils within hours at micromolar concentrations. However, no apparent IAPP amyloid aggregates are observed in healthy individuals where IAPP is stored in β-cell granules at milimolar concentrations for hours before being secreted to the blood stream. Therefore, physiological conditions of β-cell granules natively inhibit the amyloid aggregation of IAPP.
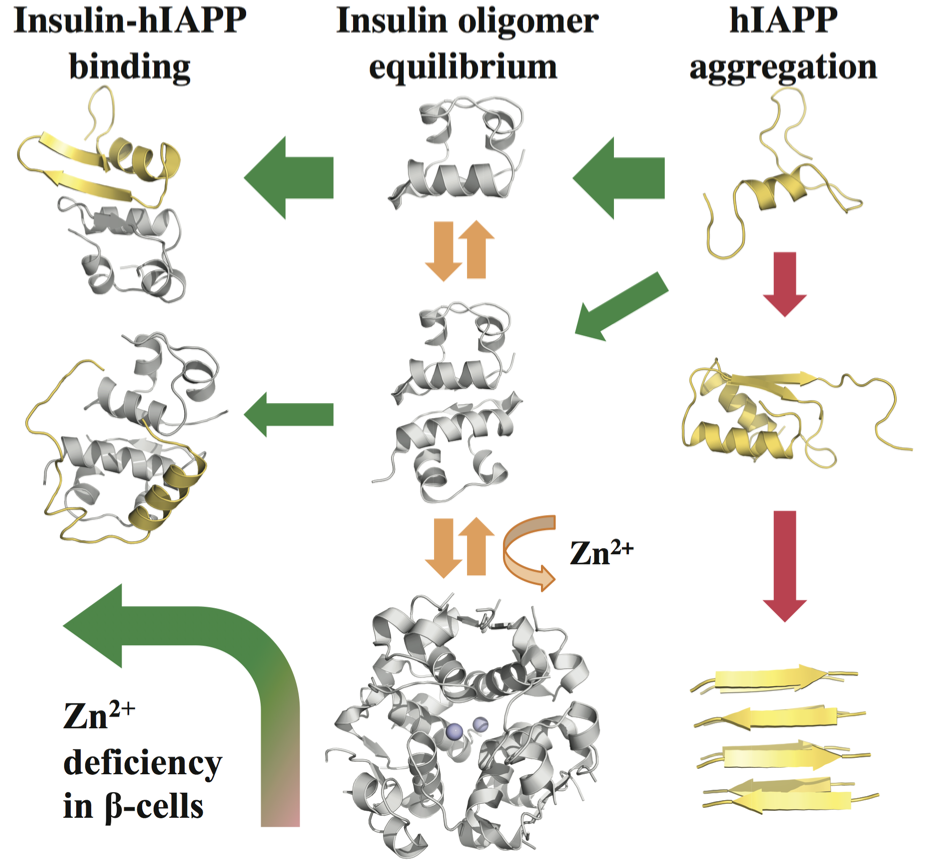 The cellular environment of β-cell granules is unique in its high concentrations of Zn2+, insulin and proinsulin c-peptide in addition to IAPP. C-peptide is the co-product of insulin synthesis, which connects insulin A- and B-chains in the precursor proinsulin and is co-secreted with insulin in equal molar. A high concentration of Zn2+, maintained by a β-cell specific zinc transporter – ZnT8, is believed to be important for the efficient storage of insulin: zinc coordinates the formation of insulin hexamers, which form crystals in the dense core of β-cell granules. ). We hypothesize that intermolecular interactions with insulin, zinc and c-peptide are important for the native inhibition of IAPP aggregation inside β-cell granules. Using state-of-the-art discrete molecular dynamics (DMD) simulations, we showed that both insulin monomers and dimers could bind IAPP monomer and inhibit IAPP self-association by competing with same amyloidogenic regions, subsequently preventing aggregation. Therefore, comparing to high zinc concentrations where insulin is insoluble in the crystal form, zinc-deficiency due to loss-of-function mutations of ZnT8 shifts insulin oligomer/crystallization equilibrium toward soluble monomers and dimers, which can efficiently inhibit IAPP aggregation and reduce T2D risk. Currently, we are trying to understand the effects of other granule molecules, including zinc, c-peptide, and their complex, on IAPP aggregation.
The cellular environment of β-cell granules is unique in its high concentrations of Zn2+, insulin and proinsulin c-peptide in addition to IAPP. C-peptide is the co-product of insulin synthesis, which connects insulin A- and B-chains in the precursor proinsulin and is co-secreted with insulin in equal molar. A high concentration of Zn2+, maintained by a β-cell specific zinc transporter – ZnT8, is believed to be important for the efficient storage of insulin: zinc coordinates the formation of insulin hexamers, which form crystals in the dense core of β-cell granules. ). We hypothesize that intermolecular interactions with insulin, zinc and c-peptide are important for the native inhibition of IAPP aggregation inside β-cell granules. Using state-of-the-art discrete molecular dynamics (DMD) simulations, we showed that both insulin monomers and dimers could bind IAPP monomer and inhibit IAPP self-association by competing with same amyloidogenic regions, subsequently preventing aggregation. Therefore, comparing to high zinc concentrations where insulin is insoluble in the crystal form, zinc-deficiency due to loss-of-function mutations of ZnT8 shifts insulin oligomer/crystallization equilibrium toward soluble monomers and dimers, which can efficiently inhibit IAPP aggregation and reduce T2D risk. Currently, we are trying to understand the effects of other granule molecules, including zinc, c-peptide, and their complex, on IAPP aggregation.
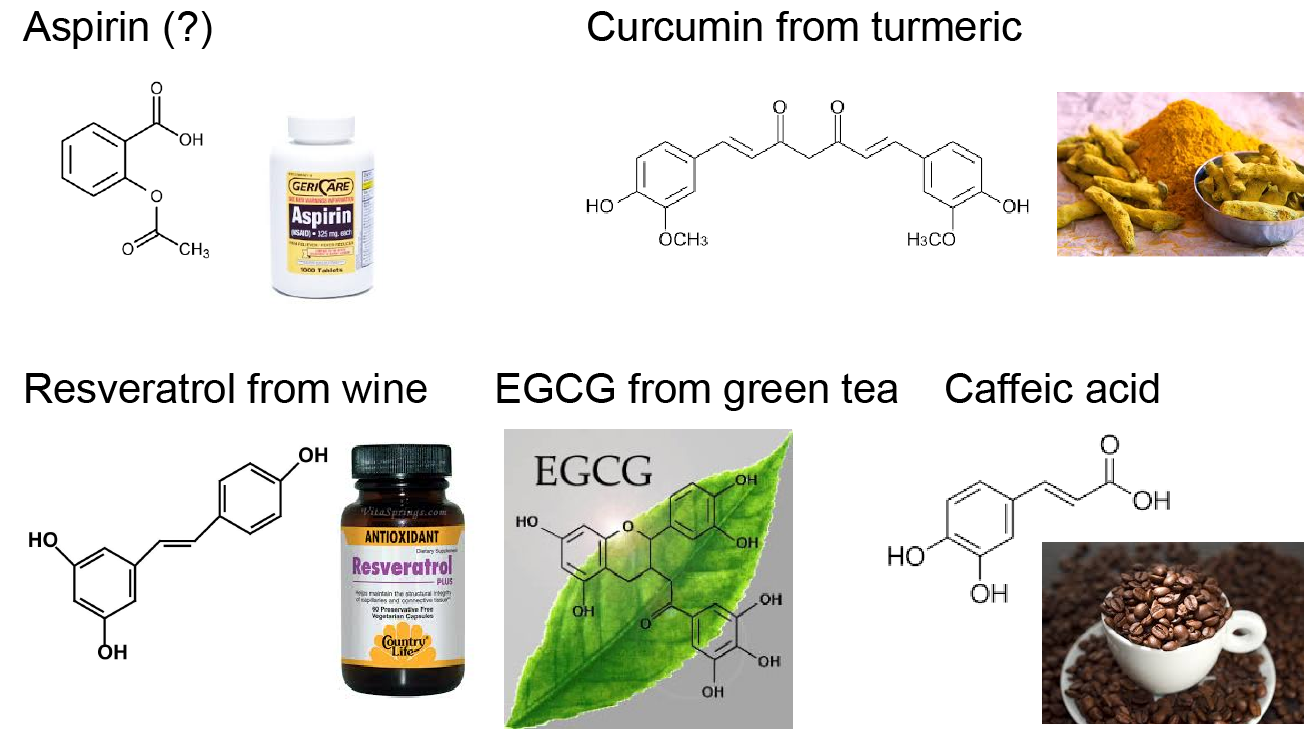 We are also actively exploring other approaches to inhibit IAPP aggregation. There are many reports about natural-occurring polyphenols, including resveratrol from grape, curcumin from turmeric, EGCG from green tea, and so on, which have inhibitory effects on IAPP aggregation. The advantage of these natural-occurring polyphenols is their low toxicity and the fact that they can be administrated for a long period of time. Understanding their mechanism is important for designing de novo anti-amyloid drugs to treat T2D. Additionally, nanomedicinal approach is also an attractive approach to serve as not only drug carriers but also as anti-amyloid agents themselves. Therefore, we are also looking for anti-amyloid nanoparticles and trying to identify their physicochemical determinants for optimal drug loading and amyloid inhibiting functions.
We are also actively exploring other approaches to inhibit IAPP aggregation. There are many reports about natural-occurring polyphenols, including resveratrol from grape, curcumin from turmeric, EGCG from green tea, and so on, which have inhibitory effects on IAPP aggregation. The advantage of these natural-occurring polyphenols is their low toxicity and the fact that they can be administrated for a long period of time. Understanding their mechanism is important for designing de novo anti-amyloid drugs to treat T2D. Additionally, nanomedicinal approach is also an attractive approach to serve as not only drug carriers but also as anti-amyloid agents themselves. Therefore, we are also looking for anti-amyloid nanoparticles and trying to identify their physicochemical determinants for optimal drug loading and amyloid inhibiting functions.
31. Z. Zhang, G. Huang, S. Gupta, E. Sargent, H. Tang, F. Ding, “Determinants for Sub-stoichiometric Inhibition of IAPP and A-Beta Amyloid Aggregations by Bri2 BRICHOS”, ACS Chemical Neuroscience, 16(6): 1150–1160 (2025) doi:10.1021/acschemneuro.4c00839
30. Z. Song, H. Tang, A. Gatch, Y. Sun and Feng Ding, “Islet Amyloid Polypeptide Fibril Catalyzes Amyloid-β Aggregation by Promoting Fibril Nucleation Rather than Direct Axial Growth”, International Journal of Biological Macromolecules (Elsevier), 279(1): 135137 (2024) doi: 10.1016/j.ijbiomac.2024.135137
29. X. Fan, X. Zhang, J. Yan, H. Xu, W. Zhao, F. Ding*, F. Huang*, Y. Sun*, “Computational Investigation of Co-Aggregation and Cross-Seeding between Aβ and hIAPP Underpinning the Crosstalk in Alzheimer’s Disease and Type-2 Diabetes”, J. Chem. Inf. Model., 64(13): 5303–5316 (2024) doi:10.1021/acs.jcim.4c00859
28. G. Huang, H. Tang, Y. Liu, C. Zhang, P. C. Ke, Y. Sun, F. Ding, “Direct Observation of Seeded Conformational Conversion of hIAPP In Silico Reveals the Mechanisms for Morphological Dependence and Asymmetry of Fibril Growth”, Journal of Chemical Information and Modeling, (63)18: 5863–5873 (2023) doi: 10.1021/acs.jcim.3c00898
27. N. Benhamou Goldfajn, H. Tang, F. Ding, “Sub-Stoichiometric Inhibition of Insulin against IAPP Aggregation is Attenuated by the Incompletely Processed N-Terminus of proIAPP”, ACS Chemical Neuroscience, 13(13): 2006–2016 (2022) doi:10.1021/acschemneuro.2c00231
26. H. Tang, Y. Sun, F. Ding, “The Hydrophobic/Hydrophilic Ratio of Amphiphilic Helix Mimetics Determines the Effects on Islet Amyloid Polypeptide Aggregation”, Journal of Chemical Information and Modeling, 62(7): 1760–1770 (2022) doi:10.1021/acs.jcim.1c01566
25. N. Andrikopoulos, Z. Song, X. Wan, A. Douek, I. Javed, C. Fu, X. Changkui, Y. Xing, F. Xin, Y. Li, A. Kakinen, K. Koppel, R. Qiao, A. Whittaker, J. Kaslin, T. Davis*, Y. Song*, F. Ding*, P.C. Ke*, “Inhibition of Amyloid Aggregation and Toxicity with Janus Iron Oxide Nanoparticles”, Chem. Mater., 33, 16, 6484–6500 (2021) doi: 10.1021/acs.chemmater.1c01947
24. Chen P, Ding F, Cai R, Javed I, Yang W, Zhang Z, Li Y, Davis TP, Ke PC, Chen C., “Amyloidosis Inhibition, a New Frontier of the Protein Corona”, Nano Today, 35:100937 (2020) doi: 10.1016/j.nantod.2020.100937
23. Koppel K, Tang H, Javed I, Parsa M, Mortimer M, Davis TP, Lin S, Chaffee AL, Ding F and Ke P, “Elevated Amyloidoses of Human IAPP and Amyloid Beta by Lipopolysaccharide and Their Mitigation by Carbon Quantum Dots”, Nanoscale, in press (2020)
22. A Nandakumar, Y Xing, R Aranha, A Faridi, A Kakinen, I Javed, K Koppel, E Pilkington, A Purcell, T Davis, P Faridi, F Ding, PC Ke, “Human Plasma Protein Corona of Aβ Amyloid and Its Impact on IAPP Cross-Seeding”, Biomacromolecules, 21, 988-998 (2020) DOI: 10.1021/acs.biomac.9b01650
21. A Faridi, Y Sun, M Mortimer, RR Aranha, A Nandakumar, Y Li, I Javed, A Kakinen, Q Fan, AW Purcell, TP Davis,* F Ding,* P Faridi,* and P Ke*, “Graphene quantum dots rescue protein dysregulation of pancreatic β-cells exposed to human islet amyloid polypeptide”, Nano Research, in press (2019)
20. A Kakinen, Y Xing, NDH Arachchi, I Javed, L Feng, A Faridi, AM Douek, Y Sun, J Kaslin, TP Davis*, MJ Higgins*, F Ding*, and P Ke*, “Single-molecular hetero-amyloidosis of human islet amyloid polypeptide”, Nano Lett, in press, (2019) Just Accepted
19. P. C. Ke, E. H. Pilkington, Y. Sun, I. Javed, A. Kakinen, G. Peng, F. Ding, . P. Davisa, “Mitigation of Amyloidosis with Nanomaterials”, Advanced Materials, in press, (2019)
18. Y. Sun, A. Kakinen, Y. Xing, P. Faridi, A. Nandakumar, A.W. Purcell, T.P. Davis, P. Ke, and F. Ding, “Amyloid self-assembly of hIAPP8-20 via the accumulation of helical oligomers, -helix to β-sheet transition, and formation of β-barrel intermediates”, Small, in press, (2019)
17. Y. Sun, A. Kakinen, Y. Xing, E.H. Pilkington, T.P. Davis, P. Ke, & F. Ding, “Nucleation of β-rich Oligomers and β-barrels in the Early Aggregation of Human Islet Amyloid Polypeptide”, BBA-Molecular Basis of Disease, 1865 (2), 434-444, DOI: 10.1016/j.bbadis.2018.11.021 (2019)
16. M. Wang, Y. Sun, X. Cao, G. Peng, I. Javed, A. Kakinen, T.P. Davis, S. Lin, J. Liu, F. Ding, and P. Ke, “Graphene Quantum Dots against Human IAPP Aggregation and Toxicity in Vivo”, Nanoscale 10, 19995, DOI: 10.1039/C8NR07180B (2018)
15. A. Faridi,Y. Sun, Y. Okazaki, G. Peng, J. Gao, A. Kakinen, P. Faridi, M. Zhao, I. Javed, A.W. Purcell, T.P. Davis, S. Lin, R. Oda, F. Ding, P. Ke, “Mitigating Human IAPP Amyloidogenesis in Vivo with Chiral Silica Nanoribbons”, Small, 14, 1802825, DOI: 10.1002/smll.201802825 (2018)
14. Pilkington E.P., Gustafsson O.J.R., Xing Y., Hernandez-Fernaud J., Zampronio C., Kakinen A., Faridi A., Ding F., Wilson P., Ke P.C. and Davis T.P., “Profiling the Serum Protein Corona of Fibrillar Human Islet Amyloid Polypeptide”, ACS Nano, in press (2018)
13. A. Kakinen, J. Adamcik, B. Wang, X. Ge, R. Mezzenga, T.P. Davis, F. Ding, and P. Ke, “Nanoscale inhibition of polymorphic and ambidextrous IAPP amyloid aggregation with small molecules”, Nano Research, in press (2017)
12. I. Javed, Y. Sun, J. Adamcik, B. Wang, A. Kakinen, E. Pilkington, F. Ding, R. Mezzenga, T. Davis, Thomas; P. Ke, “Co-fibrillization of pathogenic and functional amyloid proteins with gold nanoparticles against amyloidogenesis”, Biomacromolecules, in press (2017) DOI: 10.1021/acs.biomac.7b01359
11. Y. Xing, E. H. Pilkington, M. Wang, C. Nowell, A. Kakinen, Y. Sun, B. Wang, T. P. Davis, F. Ding and P. C. Ke, “Lysophosphatidylcholine modulates the aggregation of human islet amyloid polypeptide”, Phys. Chem. Chem. Phys., in press, 2017, DOI: 10.1039/C7CP06670H
10. E. Pilkington, M. Lai, X. Ge, W. Stanley, B. Wang, M. Wang, A. Kakinen, M. Sani, M. Whittaker, E. Gurzov, F. Ding, J. Quinn, T. Davis, P. Ke, “Star Polymers Reduce IAPP Toxicity via Accelerated Amyloid Aggregation”, Biomacromolecules, in press (2017)
9. Y. Sun, B. Wang, X. Ge and F. Ding, “Distinct Oligomerization and Fibrillization Dynamics of Amyloid Core Sequences of Amyloid-beta and Islet Amyloid Polypeptide”, PCCP, in press (2017)
8. X. Ge, A. Kakinen, E.N. Gurzov, W. Yang, L. Pang, E.H. Pilkington, P. Govindan-Nedumpully, P. Chen, F. Separovic, T.P. Davis, P. C. Ke, and F. Ding, “Zinc-coordination and C-peptide complexation: a potential mechanism for the endogenous inhibition of IAPP aggregation”, Chem. Comm., in press (2017) [link]
7. P.C. Ke, M. Sani, F. Ding, A. Kakinen, I. Javed, F. Separovic, T. P. Davis, and R. Mezzenga, Implications of Peptide Assemblies in Amyloid Diseases, Chem. Soc. Rev., in press (2017)
6. Pilkington E.H., Xing Y., Wang B., Kakinen A., Wang M., Davis T.P., Ding F., Ke P.C., “Effects of Protein Corona on IAPP Amyloid Aggregation, Fibril Remodelling, and Cytotoxicity”, Scientific Reports, in press (2017)
5. E.N. Gurzov, B. Wang, E.H. Pilkington, P. Chen,A. Kakinen, W.J. Stanley, S.A. Litwak, E.G. Hanssen,T.P. Davis, F. Ding, and P.Chun Ke, “Inhibition of hIAPP Amyloid Aggregation and Pancreatic β-cell Toxicity by OH-terminated PAMAM Dendrimer”, Small, in press (2016)
4. P. Nedumpully-Govindan, E.N. Gurzov, P. Chen, E.H. Pilkington, W.J. Stanley, S.A. Litwak, T.P. Davis, P.C. Ke, and F. Ding, “Graphene Oxide Inhibits hIAPP Amyloid Fibrillation and Toxicity in Insulin-Producing NIT-1 Cells”, PCCP, 18:94-100 (2016) [download]
3. P. Nedumpully-Govindan, A. Kakinen, E.H. Pilkington, T.P. Davis, P.C. Ke and F. Ding, “Stabilizing Off-pathway Oligomers by Polyphenol Nanoassemblies for IAPP Aggregation Inhibition”, Scientific Reports, in press (2015)
2. P. Nedumpully-Govindan, Y. Yang, R. Andorfer, W. Cao, and F. Ding, “Promotion or Inhibition of IAPP Aggregation by Zinc Coordination Depends on Its Relative Concentration”, Biochemistry, in press (2015)
1. Nedumpully-Govindan P. and Ding F., “Inhibition of IAPP aggregation by insulin depends on the insulin oligomeric state regulated by zinc ion concentration”, Scientific Reports 5, (2015)
