External publication lists: Pubmed, Google Scholar, Web of Science












205. F. Huang, Y. Zhang, X. Zhang, J. Xu, C. Wang, X. Huang, S. Liu*, F. Ding*, Y. Sun*, “Structural and energetic effects of disulfide mediation on the conformational dynamics of human calcitonin”, International Journal of Biological Macromolecules (Elsevier), 338(2): 149880 (2026) doi: 10.1016/j.ijbiomac.2025.149880
204. Z. Zhang, L. Hayes, T. Hou, F. Ding, “Parallel In-Register Contact Propensity Predicts the Amyloidogenicity of ADan and ABri in Familial Dementias”, International Journal of Biological Macromolecules (Elsevier), 338(1): 149627 (2026) doi: 10.1016/j.ijbiomac.2025.149627
203. K. Bhandari, Y. Sun, H. Tang, P. Ke, F. Ding, “A Global Thermodynamic-Kinetic Model Capturing the Hallmarks of Liquid-Liquid Phase Separation and Amyloid Aggregation”, Cell Reports Physical Science, 103031 (2025)doi: 10.1016/j.xcrp.2025.103031
202. Z. Song, K. Bhandari, T. Hou, F. Ding, “APOE genotype difference in the biphasic modulation of amyloid-β aggregation by direct binding and lowering the nucleation barrier”, Biomacromolecules, 26(12): 8494–8507 (2025) doi: 10.1021/acs.biomac.5c01323
201. D.Y. Zhang, J. Wang, G. Huang, M. Dokholyan, S. Willcox, J. Griffith, F. Ding, N.V. Dokholyan, “Apolipoprotein E (APOE) regulates the transport of monosialotetrahexosylganglioside (GM1)”, Journal of Biological Chemistry, 301(11): 110778 (2025) doi: 10.1016/j.jbc.2025.110778
200. Z. Lv, H. Xu, Y. Zhang, H. Tang, F. Ding, F. Huang, Y. Sun, “Conformational Ensemble Dynamics of Intrinsically Disordered Full-Length α- and β-Synuclein Monomers”, J Chem Inf Model., 65(17):9261-9273 (2025) doi: 10.1021/acs.jcim.5c01602.
199. H. Tang, Y. Sun, L. Wang, P.C. Ke, F. Ding, “Emergence of Compact Oligomers inside the Small-World Network of TDP-43 Condensates”, J Phys Chem Lett. 16(31):7797-7806 (2025) doi: 10.1021/acs.jpclett.5c01627.
198. X. Zhang, H. Xu, H. Tang, Z. Lv, Y. Zou, F. Huang, F. Ding*, and Y. Sun*, “The Glycine-Rich Region as a Flexible Molecular Glue Promoting hPrP106–145 Aggregation into β-Sheet Structures”, JCIM, 65(13): 7054–7064 (2025) doi: 10.1021/acs.jcim.5c00785
197. Y. Wang, G. Huang, X. Liang, N. Andrikopoulos, H. Tang, F. Ding*, P. C. Ke*, Y. Li*, “Microglial Clearance of Alzheimer’s Amyloid-Beta Obstructed by Nanoplastics”, Environmental Science: Nano, 12, 3247-3260 (2025) doi: 10.1039/D5EN00291E
196. Y. Sun, N. Andrikopoulos, G. Zhang, Y. Liu, X. Liang, D. Li, X. Suo, Y. Wang, Y. Li, C. Wang, Y. Li, P. C. Ke, F. Ding, “Formation of a β-Endorphin Corona Mitigates Alzheimer’s Amyloidogenesis”, Small, 21(26):e2409392 (2025) doi: 10.1002/smll.202409392
195. H. Tang, N. Andrikopoulos, Y. Li, S. Ke, Y. Sun*, F. Ding*, P.C. Ke*, “Emerging biophysical origins and pathogenic implications of amyloid oligomers”, Nature Communications, 16: 2937 (2025) doi: 10.1038/s41467-025-58335-y
194. A. Gatch, F. Ding, “Cross-interaction with amyloid-β drives pathogenic structural transformation within the amyloidogenic core region of TDP-43”, ACS Chemical Neuroscience, 16(8): 1565–1581, (2025) doi: 10.1021/acschemneuro.5c00084
193. S. Parris, J. Lovell, F. Ding, Z. Zhang, J. Olvey, J. Olvey II, J. Schmutz, J. Grimwood, A. Sreedasyam,S. Kumar, Z. Li, P. Joshi, J. Jenkins, C. Plott, A. Stewart, J. Webber, W. Stiller, D. Jones, C. Saski, “Polyploidy-Mediated Variations in Glutamate Receptor Proteins Linked to Fusarium Wilt Resistance in Upland Cotton”, The Plant Journal, 122(1): e70125 (2025) doi: 10.1111/tpj.70125
192. Z. Zhang, G. Huang, S. Gupta, E. Sargent, H. Tang, F. Ding, “Determinants for Sub-stoichiometric Inhibition of IAPP and A-Beta Amyloid Aggregations by Bri2 BRICHOS”, ACS Chemical Neuroscience, 16(6): 1150–1160 (2025) doi:10.1021/acschemneuro.4c00839
191. X. Liang, G. Huang, Y. Wang, N. Andrikopoulos, H. Tang, F. Ding*, Y. Li*, P.C. Ke*, “Polystyrene Nanoplastics Hitch-Hike the Gut-Brain Axis to Exacerbate Parkinson’s Pathology”, ACS Nano, 19(5): 5475–5492 (2025) doi: 10.1021/acsnano.4c13914
190. H. Xu, X. Zhang, Z. Lv, F. Huang, Y. Zou, C. Wang, F. Ding*, and Y. Sun*, “Computational exploration of the self-aggregation mechanisms of phenol-soluble modulins β1 and β2 in Staphylococcus aureus biofilms”, Colloids Surf B Biointerfaces, 248: 114498 (2025) doi: 10.1016/j.colsurfb.2025.114498
189. R. K. Goutam, G. Huang, E. Medina, F. Ding, W. J. Edenfield, and H. Sanabria, “Impact of Frequent ARID1A Mutations on Protein Stability: Insights into Cancer Pathogenesis”, Scientific Reports, 15: 3072 (2025) doi: 10.1038/s41598-025-87103-7
188. F. Huang, X, Fan, H. Xu, Z. Lv, Y. Zou, J. Lian, F. Ding*, Y. Sun*, “Computational insights into the aggregation mechanism of human calcitonin”, International Journal of Biological Macromolecules (Elsevier), 294: 139520 (2025) doi: 10.1016/j.ijbiomac.2025.139520
187. Bu F, Adam Y, Adamiak RW, Antczak M, de Aquino BRH, Badepally NG, Batey RT, Baulin EF, Boinski P, Boniecki MJ, Bujnicki JM, Carpenter KA, Chacon J, Chen SJ, Chiu W, Cordero P, Das NK, Das R, Dawson WK, DiMaio F, Ding F, Dock-Bregeon AC, Dokholyan NV, Dror RO, Dunin-Horkawicz S, Eismann S, Ennifar E, Esmaeeli R, Farsani MA, Ferré-D’Amaré AR, Geniesse C, Ghanim GE, Guzman HV, Hood IV, Huang L, Jain DS, Jaryani F, Jin L, Joshi A, Karelina M, Kieft JS, Kladwang W, Kmiecik S, Koirala D, Kollmann M, Kretsch RC, Kurciński M, Li J, Li S, Magnus M, Masquida B, Moafinejad SN, Mondal A, Mukherjee S, Nguyen THD, Nikolaev G, Nithin C, Nye G, Pandaranadar Jeyeram IPN, Perez A, Pham P, Piccirilli JA, Pilla SP, Pluta R, Poblete S, Ponce-Salvatierra A, Popenda M, Popenda L, Pucci F, Rangan R, Ray A, Ren A, Sarzynska J, Sha CM, Stefaniak F, Su Z, Suddala KC, Szachniuk M, Townshend R, Trachman RJ 3rd, Wang J, Wang W, Watkins A, Wirecki TK, Xiao Y, Xiong P, Xiong Y, Yang J, Yesselman JD, Zhang J, Zhang Y, Zhang Z, Zhou Y, Zok T, Zhang D, Zhang S, Żyła A, Westhof E, Miao Z., “RNA-Puzzles Round V: blind predictions of 23 RNA structures”, Nature Methods, 22 (2), 399-411 (2025) doi: 10.1038/s41592-024-02543-9
186. F. Huang, J. Yan, H. Xu, Y. Wang, X. Zhang, Y. Zou, J. Lian, F. Ding, Y. Sun, “Exploring the Impact of Physiological C-Terminal Truncation on α-Synuclein Conformations to Unveil Mechanisms Regulating Pathological Aggregation”, JCIM, 64(22): 8616–8627 (2024) doi: 10.1021/acs.jcim.4c01839
185. G. Huang, Z. Song, Y. Xu, Y. Sun, F. Ding, “Deciphering the Morphological Difference of Amyloid-β Fibrils in Familial and Sporadic Alzheimer’s Diseases”, JCIM, 64(20): 8024–8033 (2024) doi: 10.1021/acs.jcim.4c01471
184. H. Tang, Y. Sun, L. Wang, P.C. Ke, F. Ding, “Uncovering intermolecular interactions driving the liquid-liquid phase separation of TDP-43 low complexity domain via atomistic dimerization simulations”, JCIM, 64(19):7590-7601 (2024) doi:10.1021/acs.jcim.4c00943
183. F. Huang, J. Yan, X. Zhang, H. Xu, J. Lian, X. Yang, C. Wang*, F. Ding*, Y. Sun*, “Computational Insights into the Aggregation Mechanism and Amyloidogenic Core of Aortic Amyloid Medin Polypeptide”, Colloids and Surfaces B: Biointerfaces, 244: 114192(2024) doi:10.1016/j.colsurfb.2024.114192
182. Z. Song, H. Tang, A. Gatch, Y. Sun and Feng Ding, “Islet Amyloid Polypeptide Fibril Catalyzes Amyloid-β Aggregation by Promoting Fibril Nucleation Rather than Direct Axial Growth”, International Journal of Biological Macromolecules (Elsevier), 279(1): 135137 (2024) doi: 10.1016/j.ijbiomac.2024.135137
181. A. Gatch and F. Ding, “TDP-43 Promotes Amyloid-beta Toxicity by Delaying Fibril Maturation via Direct Molecular Interaction”, ACS Chemical Neuroscience, 15(15): 2936–2953 (2024) doi: 10.1021/acschemneuro.4c00334
180. X. Fan, X. Zhang, J. Yan, H. Xu, W. Zhao, F. Ding*, F. Huang*, Y. Sun*, “Computational Investigation of Co-Aggregation and Cross-Seeding between Aβ and hIAPP Underpinning the Crosstalk in Alzheimer’s Disease and Type-2 Diabetes”, J. Chem. Inf. Model., 64(13): 5303–5316 (2024) doi:10.1021/acs.jcim.4c00859
179. F. Huang, J. Huang, J. Yan, Y. Liu, J. Lian, Q. Sun, F. Ding, Y. Sun,”Molecular Insights into the Effects of F16L and F19L Substitutions on the Conformation and Aggregation Dynamics of Human Calcitonin”, J. Chem. Inf. Model., 64, 11, 4500–4510 (2024) doi:10.1021/acs.jcim.4c00553
178. A. Nandakumar, H. Tang, N. Andrikopoulos, J. F. Quinn, F. Ding,* P. C. Ke* and Y. Li*, “Controlling nanoparticle-induced endothelial leakiness with the protein corona”, Nanoscale, 6(19):9348-9360 (2024) doi:10.1039/D4NR01311E
177. Y. Wang, X. Liang, N. Andrikopoulos, H. Tang, F. He, X. Yin, Y. Li, F. Ding, G. Peng, M. Mortimer, P.C. Ke, “Remediation of Metal Oxide Nanotoxicity with a Functional Amyloid”, Advanced Science, 11(23):2310314 (2024) doi:10.1002/advs.202310314
176. A. Chaari, N. Saikia, P. Paul, M. Yousef, F. Ding, M. Ladjimi, “Experimental and computational investigation of the effect of Hsc70 structural variants on inhibiting amylin aggregation”, Biophysical Chemistry, 309:107235 (2024) doi:10.1016/j.bpc.2024.107235
175. F. Huang, X. Fan, Y. Wang, Y. Zou, J. Lian, C. Wang, F. Ding*, Y. Sun*, “Computational insights into the cross-talk between medin and Aβ: implications for age-related vascular risk factors in Alzheimer’s disease”, Brief. Bioinform.,25(2):bbad526 (2024) doi:10.1093/bib/bbad526
174. Y. Li, N. Ni, M. Lee, W. Wei, N. Andrikopoulos, A. Kakinen, T. P. Davis, Y. Song*, F. Ding*, D. T. Leong* and P. Ke*, “Endothelial leakiness elicited by amyloid protein aggregation”, Nature Communications,15, Article number: 613 (2024) doi:10.1038/s41467-024-44814-1
173. J. Yan, Y. Wang, X. Fan, Y. Zou, F. Ding*, F. Huang*, Y. Sun*, “Deciphering the influence of Y12L and N17H substitutions on the conformation and oligomerization of human calcitonin”, Soft Matter, 20:693-703 (2024) doi:10.1039/d3sm01332d
172. F. Huang, Y. Liu, Y. Wang, J. Xu, J. Lian, Y. Zou, C. Wang*, F. Ding* and Y. Sun*, “Co-aggregation of α-synuclein with Amyloid-β Stabilizes β-sheet-rich Oligomers and Enhances the Formation of β-barrels”, Physical Chemistry Chemical Physics, 25(46): 31604-31614 (2023) doi:10.1039/D3CP04138G
171. X. Liang, N. Andrikopoulos, H. Tang, Y. Wang, F. Ding* and P.C. Ke*, “Nanoplastic stimulates the amyloidogenesis of Parkinson’s alpha-synuclein NACore”, Small, 20(14):e2308753 (2023) doi:10.1002/smll.202308753
170. F. Huang, X. Fan, Y. Wang, C. Wang, Y. Zou, J. Lian*, F. Ding*, Y. Sun*, “Unveiling Medin Folding and Dimerization Dynamics and Conformations via Atomistic Discrete Molecular Dynamics Simulations”, Journal of Chemical Information and Modeling, 63(20): 6376–6385 (2023) doi: 10.1021/acs.jcim.3c01267
169. G. Huang, H. Tang, Y. Liu, C. Zhang, P. C. Ke, Y. Sun, F. Ding, “Direct Observation of Seeded Conformational Conversion of hIAPP In Silico Reveals the Mechanisms for Morphological Dependence and Asymmetry of Fibril Growth”, Journal of Chemical Information and Modeling, (63)18: 5863–5873 (2023) doi: 10.1021/acs.jcim.3c00898
168. S. Cao, Z. Song, J. Rong, N. Andrikopoulos, X. Liang, Y. Wang, G. Peng*, F. Ding*, P. C. Ke*, Spike Protein Fragments Promote Alzheimers Amyloidogenesis, ACS Applied Materials & Interfaces, 15(34): 40317–40329 (2023) doi: 10.1021/acsami.3c09815
167. Z. Zhang, G. Huang, Z. Song, A.J. Gatch, F. Ding , “Amyloid Aggregation and Liquid-Liquid Phase Separation from the Perspective of Phase Transitions”, J. Phys. Chem. B, 127(28): 6241–6250 (2023) doi: 10.1021/acs.jpcb.3c01426
166. Y. Wang, J. Xu, J. Yan, X. Fan, G. Wei, C. Wang, F. Ding*, Y. Sun*, “SEVI Inhibits Aβ Amyloid Aggregation by Capping the β-Sheet Elongation Edges”, Journal of Chemical Information and Modeling, 63(11):3567-3578 (2023) doi: 10.1021/acs.jcim.3c00414
165. Huang F., Wang Y., Zhang Y., Wang C., Lian J., Ding F.*, Sun Y.*, “Dissecting the Self-assembly Dynamics of Imperfect Repeats in α-Synuclein”, Journal of Chemical Information and Modeling, 63(11):3591-3600 (2023) doi: 10.1021/acs.jcim.3c00533
164. Song Z., Gatch A.J., Sun, Y. and Ding, F., “Differential binding and conformational dynamics of tau microtubule-binding repeats with a preformed amyloid-β fibril seed”, ACS Chemical Neuroscience, 14(7):1321-1330 (2023) doi: 10.1021/acschemneuro.3c00014
163. Zheng C., Wei Y., Zhang P., Xu L., Zhang Z., Lin K., Hou J., Lv X., Ding Y., Chiu Y., Jain A., Islam N., Malovannaya A., Wu Y., Ding F., Xu H., Sun M., Chen X., and Chen Y., “CRISPR/Cas9 screen uncovers functional translation of cryptic lncRNA-encoded open reading frames in human cancer”, Journal of Clinical Investigation, 133(5):e159940 (2023) doi: 10.1172/JCI159940
162. Andrikopoulos N., Li Y., Nandakumar A., Quinn J., Davis T., Ding F.*, Saikia N.*, Ke P.C.*, “Zinc-Epigallocatechin-3-gallate Network-Coated Nanocomposites against the Pathogenesis of Amyloid-Beta”, ACS Applied Materials & Interfaces, 15, 6, 7777–7792 (2023) doi:10.1021/acsami.2c20334
161. S. Basak, N. Saikia, D. Kwun, U. B. Choi, F. Ding, M. E. Bowen, “Different Forms of Disorder in NMDA-Sensitive Glutamate Receptor Cytoplasmic Domains Are Associated with Differences in Condensate Formation”, Biomolecules, 13(1):4 doi:10.3390/biom13010004(2023)
160. Y. Liu, Y. Wang, Y. Zhang, Y. Zou, G. Wei, F. Ding* and Y. Sun*, “Structural perturbation of monomers determines the amyloid aggregation propensity of calcitonin variants”, Journal of Chemical Information and Modeling, 63, 1, 308–320 doi:10.1021/acs.jcim.2c01202:(2023)
159. Y. Zhang, Y. Wang, Y. Liu, G. Wei, F. Ding, Y. Sun, “Molecular Insights into the Misfolding and Dimerization Dynamics of the Full-length α-synuclein from Atomistic Discrete Molecular Dynamics Simulations”, ACS Chemical Neuroscience, 13, 21, 3126–3137 doi:10.1021/acschemneuro.2c00531(2022)
158. J. He, L. Zhou, G. Huang, J. Shen, W. Chen, C. Wang, A. Kim, Z. Zhang, W. Cheng, S. Dai, F. Ding,* and P. Chen*, “Enhanced Label-Free Nanoplasmonic Cytokine Detection in SARS-CoV-2 Induced Inflammation Using Rationally Designed Peptide Aptamer”, ACS Applied Materials & Interfaces, 14, 43, 48464–48475 doi:10.1021/acsami.2c14748 (2022)
157. Y. Xing, N. Andrikopoulos, Z. Zhang, Y. Sun, P.C. Ke, F. Ding, “Modulating nanodroplet formation en route to fibrillization of amyloid peptides with designed flanking sequences”, Biomacromolecules, 23(10):4179-4191, doi:10.1021/acs.biomac.2c00642 (2022)
156. G.L. Hamilton, N. Saikia, S. Basak, F.S. Welcome, F. Wu, J. Kubiak, C. Zhang, Y. Hao, C.A.M. Seidel, F. Ding*, H. Sanabria* and M.E. Bowen*, “Fuzzy Supertertiary Interactions within PSD-95 Enable Ligand Binding”, eLife, 11: e77242, doi:10.7554/eLife.77242 (2022) The accompanied Insight article.
155. Gumna J, Antczak M, Adamiak RW, Bujnicki JM, Chen SJ, Ding F, Ghosh P, Li J, Mukherjee S, Nithin C, Pachulska-Wieczorek K, Ponce-Salvatierra A, Popenda M, Sarzynska J, Wirecki T, Zhang D, Zhang S, Zok T, Westhof E, Miao Z, Szachniuk M, Rybarczyk A., “Computational Pipeline for Reference-Free Comparative Analysis of RNA 3D Structures Applied to SARS-CoV-2 UTR Models”, International Journal of Molecular Sciences, doi: 10.3390/ijms23179630 (2022)
154. W. Wei, Y. Li, M. Lee, N. Andrikopoulos, S. Lin, C. Chen, D. Leong, F. Ding, Y. Song, and P. Ke, “Anionic Nanoplastic Exposure Induces Endothelial Leakiness”, Nature Communications, 13: 4757, doi:10.1038/s41467-022-32532-5 (2022) *Featured in Editors’ Highlights: “Translational and Clinical Research” of Nat Comm.
153. Y. Wang, Y. Liu, Y. Zhang, G. Wei, F. Ding, Y. Sun, “Molecular Insights into the Oligomerization Dynamics and Conformations of Amyloidogenic and Non-Amyloidogenic Amylin from Discrete Molecular Dynamics Simulations”, Physical Chemistry Chemical Physics, 24, 21773-21785, doi:10.1039/D2CP02851D (2022)
152. Y. Liu, Y. Wang, C. Tong, G. Wei, F. Ding*, Y. Sun*, “Molecular Insights into the Self-assembly of Block Copolymer Suckerin Polypeptides into Nanoconfined β-sheets”, Small, 18(34): 2202642, doi: 10.1002/smll.202202642 (2022)
151. N. Benhamou Goldfajn, H. Tang, F. Ding, “Sub-Stoichiometric Inhibition of Insulin against IAPP Aggregation is Attenuated by the Incompletely Processed N-Terminus of proIAPP”, ACS Chemical Neuroscience, 13(13): 2006–2016 (2022) doi:10.1021/acschemneuro.2c00231
150. J. Ren, K. Velonia, N. Andrikopoulos, H. Tang, F. Ding, P. C. Ke, C. Chen, “Chemical and Biophysical Signatures of the Protein Corona in Nanomedicine”, Journal of The American Chemical Society, 144(21): 9184–9205 (2022) doi:10.1021/jacs.2c02277
149. H. Tang, Y. Sun, F. Ding, “The Hydrophobic/Hydrophilic Ratio of Amphiphilic Helix Mimetics Determines the Effects on Islet Amyloid Polypeptide Aggregation”, Journal of Chemical Information and Modeling, 62(7): 1760–1770 (2022) doi:10.1021/acs.jcim.1c01566
148. H. Tang, Y. Li, A. Kakinen, N. Andrikopoulos, Y. Sun, E. Kwak, T. P. Davis, F. Ding* and P. C. Ke*, “Graphene Quantum Dots Obstruct the Membrane Axis of Alzheimer’s Amyloid Beta”, Physical Chemistry Chemical Physics, 24, 86-97 (2022) doi: 10.1039/D1CP04246G
147. A. Nandakumar, W. Wei , G. Siddiqui, H. Tang, Y. Li , A. Kakinen, X. Wan, K. Koppel, S. Lin, T. Davis, D. Leong, D. Creek, F. Ding, Y. Song, P. C. Ke, “Dynamic Protein Corona of Gold Nanoparticles with An Evolving Morphology”, ACS Appl. Mater. Interfaces, 13, 48, 58238–58251 (2021) doi: 10.1021/acsami.1c19824
146. Y. Liu, Y. Zhang, Y. Sun and F. Ding, “A Buried Glutamate in the Cross-β Core Renders β-endorphin Fibrils Reversible”, Nanoscale, 13, 19593-19603 (2021) doi:10.1039/D1NR05679D
145. N. Saikia, I. S. Yanez-Orozco, R. Qiu, P. Hao, S. Milikisiyants, E. Ou, G. L. Hamilton, K. R. Weninger, T. Smirnova, H. Sanabria, and F. Ding, “Integrative structural dynamics probing of the conformational heterogeneity in synaptosomal-associated protein 25”, Cell Reports Physical Science 2:100616 (2021) doi:10.1016/j.xcrp.2021.100616
144. N. Kolimi, A. Pabbathi, N. Saikia, F. Ding, H. Sanabria, J. Alper, “Out-of-Equilibrium Biophysical Chemistry: The Case for Multidimensional, Integrated Single-Molecule Approaches”, J. Phys. Chem. B, 125, 37, 10404–10418 (2021) doi:10.1021/acs.jpcb.1c02424
143. M. Lee,† N. Ni,† H. Tang,† Y. Li,† W. Wei, A. Kakinen, X. Wan, T. P. Davis, Y. Song,* D. T. Leong,* F. Ding* and P. C. Ke*, “A Framework of Paracellular Transport via Nanoparticles-Induced Endothelial Leakiness”, Advanced Science, 2102519 (2021) doi:10.1002/advs.202102519
142. N. Andrikopoulos, Z. Song, X. Wan, A. Douek, I. Javed, C. Fu, X. Changkui, Y. Xing, F. Xin, Y. Li, A. Kakinen, K. Koppel, R. Qiao, A. Whittaker, J. Kaslin, T. Davis*, Y. Song*, F. Ding*, P.C. Ke*, “Inhibition of Amyloid Aggregation and Toxicity with Janus Iron Oxide Nanoparticles”, Chem. Mater., 33, 16, 6484–6500 (2021) doi: 10.1021/acs.chemmater.1c01947
141. H. Simon-Baram, D. Kleiner, F. Shmulevich, R. Zarivach, R. Zalk, H. Tang, F. Ding, S. Bershtein, “SAMase of bacteriophage T3 inactivates E. coli’s methionine S-adenosyltransferase by forming hetero-polymers”, mBio, Vol. 12, No. 4 (2021) doi: 10.1128/mBio.01242-21
140. Y. Li, H. Tang, H. Zhu, A. Kakinen, D. Wang, N. Andrikopoulos, Y. Sun, A. Nandakumar, E. Kwak, T. Davis, D. Leong, F. Ding, P. C. Ke, “Ultrasmall Molybdenum Disulfide Quantum Dots Cage Alzheimer’s Amyloid Beta to Restore Membrane Fluidity”, ACS Appl. Mater. Interfaces, 13(25): 29936–29948 (2021) doi: 10.1021/acsami.1c06478
139. H. He, Y. Liu, Y. Sun, F. Ding, “The Misfolding and Self-assembly Dynamics of Microtubule-binding Repeats of the Alzheimer-related Protein Tau”, J. Chem. Info. Model, 61(6): 2916–2925 (2021) doi: 10.1021/acs.jcim.1c00217
138. J. Ma, N. Saikia, S. Godar, G.L. Hamilton, F. Ding*, J. Alper*, H. Sanabria*, “Ensemble Switching Unveils a Kinetic Rheostat Mechanism of the Eukaryotic Thiamine Pyrophosphate Riboswitch”, RNA, 27(7): 771–790 (2021) doi: 10.1261/rna.075937.120
137. Y. Sun, A. Kakinen, X. Wan, N. Moriarty, C.P.J. Hunt, Y. Li, N. Andrikopoulos, A. Nandakumar, T.P. Davis, C.L. Parish, Y. Song, P. C. Ke and F. Ding, “Spontaneous Formation of β-sheet Nano-barrels during the Early Aggregation of Alzheimer’s Amyloid Beta”, Nano Today, 38, 101125 (2021) doi: 10.1016/j.nantod.2021.101125
136. Y. Sun, J. Huang, X. Duan and F. Ding, “Direct Observation of β-barrel Intermediates in the Self-assembly of Toxic SOD128-38 and Absence in Non-toxic Glycine Mutants”, J. Chem. Info. Model, 61, 2, 966–975 (2021) doi: 10.1021/acs.jcim.0c01319
135. S. Basak, N. Saikia, L. Dougherty, Z. Guo, F. Wu, F. A. Mindlin, J. W. Lary, J. L. Cole, F. Ding & M. E. Bowen, “Probing interdomain linkers and protein supertertiary structure in vitro and in live cells with fluorescent protein resonance energy transfer”, J. Mol. Biol., 433(5): 166793
(2021) doi: doi.org/10.1016/j.jmb.2020.166793
134. Y. Xing, A. Nandakumar, A. Kakinen, Y. Sun, T.P. Davis, P. C. Ke, and F. Ding, “Amyloid Aggregation under the Lens of Liquid-Liquid Phase Separation”, J. Phys. Chem. Lett., 12, XXX, 368–378 (2021)doi: doi.org/10.1021/acs.jpclett.0c02567
133. Y. Li, H. Tang, N. Andrikopoulos, I. Javed, L. Cecchetto, A. Nandakumar, A. Kakinen, T.P. Davis, F. Ding, and P. Ke, “The Membrane Axis of Alzheimer’s Nanomedicine”, Advanced NanoBiomed Research, 1, 2000040 (2021) doi: doi.org/10.1002/anbr.202000040
132. Chen P, Ding F, Cai R, Javed I, Yang W, Zhang Z, Li Y, Davis TP, Ke PC, Chen C., “Amyloidosis Inhibition, a New Frontier of the Protein Corona”, Nano Today, 35:100937 (2020) doi: 10.1016/j.nantod.2020.100937
131. Y. Sun, F. Ding, “αB-Crystallin Chaperone Inhibits Aβ Aggregation by Capping the β-Sheet-Rich Oligomers and Fibrils”, J. Phys. Chem. B, 124:10138-10146 (2020) doi: 10.1021/acs.jpcb.0c07256.
130. I Javed,Z Zhang, J Adamcik, N Andrikopoulos, Y Li, DE Otzen, S Lin, R Mezzenga, TP Davis, F Ding* and P Ke*, “Accelerated Amyloid Beta Pathogenesis by Bacterial Amyloid FapC”, Advanced Science, 7, 2001299 (2020) doi: doi.org/10.1002/advs.202001299
– Featured as the Cover.
129. K. Koppel, H. Tang, I. Javed, M. Parsa, M. Mortimer, T.P. Davis, S. Lin, A.L. Chaffee, F. Ding* and P.C. Ke*, “Elevated Amyloidoses of Human IAPP and Amyloid Beta by Lipopolysaccharide and Their Mitigation by Carbon Quantum Dots”, Nanoscale, 12, 12317-12328 (2020) doi: 10.1039/D0NR02710C
128. Community/Group Publication — Miao Z, Adamiak RW, Antczak M, Boniecki MJ, Bujnicki JM, Chen SJ, Cheng CY, Cheng Y, Chou FC, Das R, Dokholyan NV, Ding F, Geniesse C, Jiang Y, Joshi A, Krokhotin A, Magnus M, Mailhot O, Major F, Mann TH, Piatkowski P, Pluta R, Popenda M, Sarzynska J, Sun L, Szachniuk M, Tian S, Wang J, Wang J, Watkins AM, Wiedemann J, Xiao Y, Xu X, Yesselman JD, Zhang D, Zhang Y, Zhang Z, Zhao C, Zhao P, Zhou Y, Zok T, Zyla A, Ren A, Batey RT, Golden BL, Huang L, Lilley DM, Liu Y, Patel DJ, Westhof E., “RNA-Puzzles Round IV: 3D Structure Predictions of Four Ribozymes and Two Aptamers”, RNA, 26(8): 982–995 (2020) doi: 10.1261/rna.075341.120
127. Y. Xing, Y. Sun, B. Wang, & F. Ding, “Morphological Determinants of Carbon Nanomaterial-Induced Amyloid Peptide Self-Assembly”, Frontiers in Chemistry, 8: 160 (2020) DOI: 10.3389/fchem.2020.00160 PMCID:PMC7076083
126. Y. Sun, F. Ding, “Thermo- and pH-Responsive Fibrillization of Squid Suckerin A1H1 Peptide”, Nanoscale, 12, 6307 – 6317 (2020) DOI: 10.1039/C9NR09271D
125. A Nandakumar, Y Xing, R Aranha, A Faridi, A Kakinen, I Javed, K Koppel, E Pilkington, A Purcell, T Davis, P Faridi, F Ding, PC Ke, “Human Plasma Protein Corona of Aβ Amyloid and Its Impact on IAPP Cross-Seeding”, Biomacromolecules, 21, 988-998 (2020) DOI: 10.1021/acs.biomac.9b01650
124. Z Huma,I Javed, Z Zhang, H Bilal, Y Sun, SZ Hussain, TP Davis, DE Otzen, CB Landersdorfer, F Ding, I Hussain and PC Ke, “Nano Silver Mitigates Biofilm Formation via FapC Amyloidosis Inhibition”, Small, 1906674-1906683 (2019) DOI: 10.1002/smll.201906674
123. A Faridi, Y Sun, M Mortimer, RR Aranha, A Nandakumar, Y Li, I Javed, A Kakinen, Q Fan, AW Purcell, TP Davis,* F Ding,* P Faridi,* and P Ke*, “Graphene quantum dots rescue protein dysregulation of pancreatic β-cells exposed to human islet amyloid polypeptide”, Nano Research, 12(11), 2827–2834 (2019) DOI: 10.1007/s12274-019-2520-7
122. A Kakinen, Y Xing, NDH Arachchi, I Javed, L Feng, A Faridi, AM Douek, Y Sun, J Kaslin, TP Davis*, MJ Higgins*, F Ding*, and P Ke*, “Single-molecular hetero-amyloidosis of human islet amyloid polypeptide”, Nano Lett, 19(9), 6535-6546 (2019) DOI: 10.1021/acs.nanolett.9b02771
121. I Javed, G Peng, Y Xing, T Yu, M Zhao, A Kakinen, A Faridi, CL Parish, F Ding*, TP Davis*, P Ke* and S Lin*, “Inhibition of Amyloid Beta Toxicity in Zebrafish with A Chaperone-Gold Nanoparticle Dual Strategy”, Nature Communications, 10, 3780 (2019) DOI: 10.1038/s41467-019-11762-0
120. Y. Sun, A. Kakinen, C. Zhang, Y. Yang, A. Faridi, T. P. Davis, W. Cao, P. C. Ke and F. Ding, “Amphiphilic Surface Chemistry of Fullerenols Is Necessary for Inhibiting the Amyloid Aggregation of Alpha-Synuclein NACore”, Nanoscale, 11, 11933 – 11945 (2019) DOI: 10.1039/C9NR02407G
119. P.C. Ke, E.H. Pilkington, Y. Sun, I. Javed, A. Kakinen, G. Peng, F. Ding, T.P. Davis, “Mitigation of Amyloidosis with Nanomaterials”, Advanced Materials, 32(18):e1901690, (2020) DOI: 10.1002/adma.201901690
-Featured as the Cover story.
118. Y. Sun, A. Kakinen, Y. Xing, P. Faridi, A. Nandakumar, A.W. Purcell, T.P. Davis, P.C. Ke, and F. Ding, “Amyloid self-assembly of hIAPP8-20 via the accumulation of helical oligomers, alpha-helix to beta-sheet transition, and formation of β-barrel intermediates”, Small, 5(18):e1805166, (2019) DOI: 10.1002/smll.201805166
– Featured as the Cover.
117. Y. Sun, A. Kakinen, Y. Xing, E.H. Pilkington, T.P. Davis, P. Ke, & F. Ding, “Nucleation of β-rich Oligomers and β-barrels in the Early Aggregation of Human Islet Amyloid Polypeptide”, BBA-Molecular Basis of Disease, 1865 (2), 434-444, DOI: 10.1016/j.bbadis.2018.11.021 (2019)
116. A. Kakinen, Y. Sun, I. Javed, A. Faridi, E.H. Pilkington, P. Faridi, A.W. Purcell, R. Zhou, F. Ding, S. Lin, P. Ke, and T. P. Davis, “Physical and Toxicological Profiles of Human IAPP Amyloids and Plaques”, Science Bulletin, 64(1): 26-35 DOI: 10.1016/j.scib.2018.11.012 (2019)
115. M. Wang, Y. Sun, X. Cao, G. Peng, I. Javed, A. Kakinen, T.P. Davis, S. Lin, J. Liu, F. Ding, and P. Ke, “Graphene Quantum Dots against Human IAPP Aggregation and Toxicity in Vivo”, Nanoscale 10, 19995, DOI: 10.1039/C8NR07180B (2018)
114. A. Faridi,Y. Sun, Y. Okazaki, G. Peng, J. Gao, A. Kakinen, P. Faridi, M. Zhao, I. Javed, A.W. Purcell, T.P. Davis, S. Lin, R. Oda, F. Ding, P. Ke, “Mitigating Human IAPP Amyloidogenesis in Vivo with Chiral Silica Nanoribbons”, Small, 14, 1802825, DOI: 10.1002/smll.201802825 (2018)
113. I.Y. Orozco , F. Mindlin , J. Ma , B. Levesque , B. Wang , M. Spencer , G. Hamilton , S.R. Adariani , F. Ding*, M. Bowen*, and H. Sanabria*, “Identifying Weak Interdomain Interactions that Stabilize the Supertertiary Structure of the N-Terminal Tandem PDZ Domains of PSD-95”, Nature Communication 9: 3724, DOI: 10.1038/s41467-018-06133-0, (2018)
112. B. Wang, Y. Sun, T. Davis, P.C. Ke, Y. Wu, and F. Ding, “Understanding the Effects of PAMAM Dendrimer Size and Surface Chemistry on Serum Protein Binding with Discrete Molecular Dynamics Simulations”, ACS Sustainable Chemistry & Engineering, 6 (9),11704–11715, DOI: 10.1021/acssuschemeng.8b01959 , (2018)
111. Y. Sun, X. Ge , Y. Xing, Bo Wang, and F. Ding, “β-barrel Oligomers as Common Intermediates of Peptides Self-Assembling into Cross-β Aggregates”, Scientific Reports 8: 10353, DOI: 10.1038/s41598-018-28649-7 (2018)
110. Pilkington E.P., Gustafsson O.J.R., Xing Y., Hernandez-Fernaud J., Zampronio C., Kakinen A., Faridi A., Ding F., Wilson P., Ke P.C. and Davis T.P., “Profiling the Serum Protein Corona of Fibrillar Human Islet Amyloid Polypeptide”, ACS Nano, 12, 6066−6078 (2018) DOI: 10.1021/acsnano.8b02346
-Featured as Journal Front Cover .
109. Sun Y., Ding F., Ming D., “Nonnative Energetic Frustrations in Protein Folding at Residual Level: A Simulation Study of Homologous Immunoglobulin-like β-Sandwich Protein”, Int J Mol Sci., 19(5), pii: E1515 (2018) DOI: 10.3390/ijms19051515 .
108. X. Ge., Y. Sun and F. Ding, “Structures and Dynamics of β-barrel Oligomer Intermediates of Amyloid-beta16-22 Aggregation”, BBA Biomembranes, 1860(9): 1687-1697 (2018) DOI: 10.1016/j.bbamem.2018.03.011
107. X. Ge., Y. Yang, Y. Sun, W. Cao and F. Ding, “Islet Amyloid Polypeptide Promotes Amyloid-beta Aggregation by Binding-induced Helix-unfolding of the Amyloidogenic Core”, ACS Chem. Neurosci., 9(5):967-975 (2018)DOI: 10.1021/acschemneuro.7b00396
106. A. Kakinen, J. Adamcik, B. Wang, X. Ge, R. Mezzenga, T.P. Davis, F. Ding, and P. Ke, “Nanoscale inhibition of polymorphic and ambidextrous IAPP amyloid aggregation with small molecules”, Nano Research, 11(7): 3636–3647 (2018)[download] DOI: 10.1007/s12274-017-1930-7
105. Williams, B., Zhao, B., Tandon, A., Ding, F., Weeks, K. M., Zhang, Q., and Dokholyan, N. V. “Structure modeling of RNA using sparse NMR constraints”, Nucleic Acids Research, 45(22):12638-12647 (2017) DOI: 10.1093/nar/gkx1058
104. I. Javed, Y. Sun, J. Adamcik, B. Wang, A. Kakinen, E. Pilkington, F. Ding, R. Mezzenga, T. Davis, Thomas; P. Ke, “Co-fibrillization of pathogenic and functional amyloid proteins with gold nanoparticles against amyloidogenesis”, Biomacromolecules, 18, 4316–4322 (2017) DOI: 10.1021/acs.biomac.7b01359
103. Y. Xing, E. H. Pilkington, M. Wang, C. Nowell, A. Kakinen, Y. Sun, B. Wang, T. P. Davis, F. Ding and P. C. Ke, “Lysophosphatidylcholine modulates the aggregation of human islet amyloid polypeptide”, Phys. Chem. Chem. Phys., 19, 30627-30635, 2017, DOI: 10.1039/C7CP06670H
102. E. Pilkington, M. Lai, X. Ge, W. Stanley, B. Wang, M. Wang, A. Kakinen, M. Sani, M. Whittaker, E. Gurzov, F. Ding, J. Quinn, T. Davis, P. Ke, “Star Polymers Reduce IAPP Toxicity via Accelerated Amyloid Aggregation”, Biomacromolecules, 18 4249–4260, (2017) DOI: 10.1021/acs.biomac.7b01301
-Featured as ACS Editors’ Choice and Journal Front Cover Article
101. Y. Sun, B. Wang, X. Ge and F. Ding, “Distinct Oligomerization and Fibrillization Dynamics of Amyloid Core Sequences of Amyloid-beta and Islet Amyloid Polypeptide”, Phys. Chem. Chem. Phys., 19, 28414-28423 (2017) DOI: CP/C7CP05695H
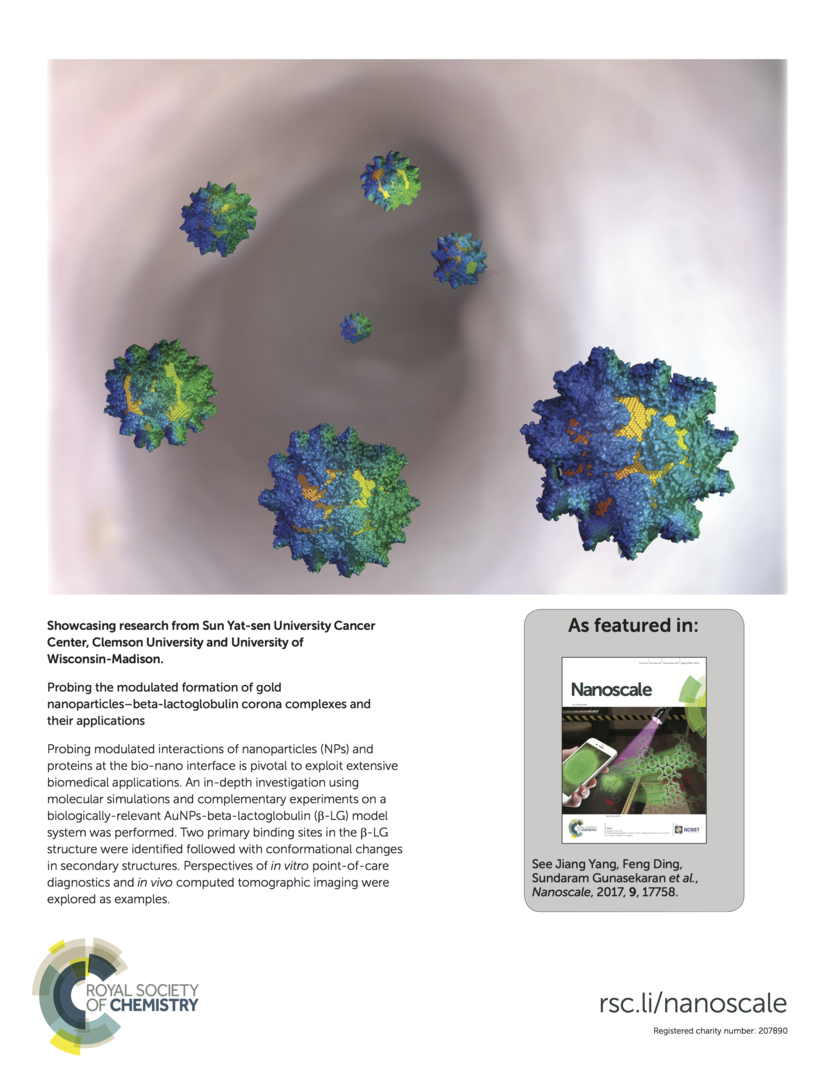
100. J. Yang, B. Wang, Y. You, W. Chang, K. Tang, Y. Wang, W. Zhang, F. Ding, S. Gunasekaran, “Probing the modulated formation of gold nanoparticle-beta lactoglobulin corona complexes and its applications”, Nanoscale, 9(45):17758-17769 (2017) DOI: 10.1039/C7NR02999C
-Featured as the back cover.
99. B. Wang, E.H. Pilkington, Y. Sun, T.P. Davis, P.C. Ke and F. Ding, “Modulating protein amyloid aggregation with nanomaterials”, Environmental Science Nano, 4, 1772-1783 (2017) DOI: 10.1039/C7EN00436B
98. X. Ge, A. Kakinen, E.N. Gurzov, W. Yang, L. Pang, E.H. Pilkington, P. Govindan-Nedumpully, P. Chen, F. Separovic, T.P. Davis, P. C. Ke, and F. Ding, “Zinc-coordination and C-peptide complexation: a potential mechanism for the endogenous inhibition of IAPP aggregation”, Chem. Comm., 53(68):9394-9397 (2017) DOI: 10.1039/C7CC04291D
-Featured as the Cover article.
97. N.K. Geitner, W. Zhao, F. Ding, W. Chen, M. R. Wiesner, “Mechanistic Insights from Discrete Molecular Dynamics Simulations of PesticideNanoparticle Interactions”, Environmental Science & Technology, 51(15):8396-8404 (2017) [link]
96. P.C. Ke, M. Sani, F. Ding, A. Kakinen, I. Javed, F. Separovic, T. P. Davis, and R. Mezzenga, “Implications of Peptide Assemblies in Amyloid Diseases”, Chem. Soc. Rev., 46:6492-6531 (2017) [download]
-Featured as the front Cover article
95. S. Lin, M. Mortimer, R. Chen, A. Kakinen, J.E. Riviere, T.P. Davis, F. Ding, and P. C. Ke, “NanoEHS beyond Toxicity – Focusing on Biocorona”, Env. Sci. Nano, 4, 1433-1454 (2017) [link][download]
-Featured as the Cover article.
94. Pilkington E.H., Xing Y., Wang B., Kakinen A., Wang M., Davis T.P., Ding F., Ke P.C., “Effects of Protein Corona on IAPP Amyloid Aggregation, Fibril Remodelling, and Cytotoxicity”, Scientific Reports, 7(1):2455 (2017) [link]
93. Community/Group Publication — Miao, Z., Adamiak, R. W., Antczak, M., Batey, R. T., Becka, A. J., Biesiada, M., Boniecki, M. J., Bujnicki, J., Chen, S. J., Cheng, C. Y., Chou, F. C., Ferré-D’Amaré A. R., Das, R., Dawson W. K., Ding, F., Dokholyan, N. V., Dunin-Horkawicz, S., Geniesse, C., Kappel, K., Kladwang, W., Krokhotin, A., Lach, G. E., Major, F., Mann, T. H., Magnus, M., Pachulska-Wieczorek, K., Patel, D. J., Piccirilli, J. A., Popenda, M., Purzycka, K. J., Ren, A., Rice, G. M., Santalcia, J. Jr., Sarzynska, J., Szachniuk, M., Tandon, A., Trausch, J. J., Tian, S., Wang, J., Weeks, K. M., Williams B. 2nd, Xiao, Y., Xu, X., Zhang, D., Zok, T., and Westhof, E. “RNA-Puzzles Round III: 3D RNA structure prediction of five riboswitches and one ribozyme”, RNA, 23(5):655-672 (2017) [link]
92. B. Wang,T. Blin, A. Käkinen, X. Ge, E.H. Pilkington, J.F. Quinn, M.R. Whittaker, T.P. Davis, P.C. Ke and F. Ding, “Brushed polyethylene glycol and phosphorylcholine for grafting nanoparticles against protein binding”, Polymer Chemistry, 7(45):6875-6879 (2016) [link][Download]
-Featured as the Cover article.
91. E. Salonen, F. Ding, and P.C. Ke, “Fate, behavior and biophysical modeling of nanoparticles in living systems” in “Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity”, Edited by B. Xing, C.D. Vecitis, & N. Senesi, Wiley-IUPAC (2016) [download]
90. Hadi-Alijanvand, H., Proctor, E. A., Ding, F., Dokholyan, N. V. and Moosavi-Movahedi, A. A. “A Hidden Aggregation-Prone Structure in the Heart of Hypoxia Inducible Factor Prolyl Hydroxylase”, Proteins: Structure, Function and Bioinformatics, 84(5): 611-623 , (2016) [link]
89. T. Blin, A. Kakinen, E.H. Pilkington, A. Ivask, F. Ding, J.F. Quinn. M.R. Whittaker, P.C. Ke and T.P. Davis, “Synthesis and In Vitro Properties of Iron Oxide Nanoparticles Grafted with Brushed Phosphorylcholine and Polyethylene Glycol”, Polymer Chemistry, 7:1931-1944 (2016) [link]
88. Nedumpully-Govindan P., Domen J., and Ding F., “CSAR Benchmark of flexible MedusaDock in affinity prediction and native-like binding pose selection”, Journal of Chemical Information and Modeling, 56(6): 1042-1052 (2016) [link]
87. E.N. Gurzov, B. Wang, E.H. Pilkington, P. Chen,A. Kakinen, W.J. Stanley, S.A. Litwak, E.G. Hanssen,T.P. Davis, F. Ding, and P.Chun Ke, “Inhibition of hIAPP Amyloid Aggregation and Pancreatic β-cell Toxicity by OH-terminated PAMAM Dendrimer”, Small, 12(12):1615–1626 (2016) [link]
86. P. Nedumpully-Govindan, E.N. Gurzov, P. Chen, E.H. Pilkington, W.J. Stanley, S.A. Litwak, T.P. Davis, P.C. Ke, and F. Ding, “Graphene Oxide Inhibits hIAPP Amyloid Fibrillation and Toxicity in Insulin-Producing NIT-1 Cells”, Phys. Chem. Chem. Phys., 18:94-100 (2016) [download]
85. P. Nedumpully-Govindan, A. Kakinen, E.H. Pilkington, T.P. Davis, P.C. Ke and F. Ding, “Stabilizing Off-pathway Oligomers by Polyphenol Nanoassemblies for IAPP Aggregation Inhibition”, Scientific Reports 6: 19463 (2016) [download]
84. S. Radic, T.P. Davis, P.C. Ke and F. Ding, “Contrasting effects of nanoparticle-protein attraction on amyloid aggregation”, RSC Advances, 5, 105489-105498 (2015)[download]
83. P. Nedumpully-Govindan, Y. Yang, R. Andorfer, W. Cao, and F. Ding, “Promotion or Inhibition of IAPP Aggregation by Zinc Coordination Depends on Its Relative Concentration”, Biochemistry, 54:7335-44 (2015)[download]
82. Wang B, Geitner NK, Davis TP, Ke PC, Ladner DL and Ding F, “Deviation from the Unimolecular Micelle Paradigm of PAMAM Dendrimers Induced by Strong Inter-Ligand Interactions”, Journal of Physical Chemistry C, 119, 19475–19484 (2015) [download]
81. Ge XW, Ke PC, Davis TP and Ding F, “A Thermodynamics Model for the Emergence of a Stripe-like Binary SAM on a Nanoparticle Surface”, Small 11(37):4894–4899 (2015) [link]
-Featured as the Cover article [link]
80. Community/Group Publication — Miao Z, Adamiak RW, Blanchet MF, Boniecki M, Bujnicki JM, Chen SJ, Cheng C, Chojnowski G, Chou FC, Cordero P, Cruz JA, Ferré-D’amaré AR, Das R, Ding F, Dokholyan NV, Dunin-Horkawicz S, Kladwang W, Krokhotin A, Lach G, Magnus M, Major F, Mann TH, Masquida B, Matelska D, Meyer M, Peselis A, Popenda M, Purzycka KJ, Serganov A, Stasiewicz J, Szachniuk M, Tandon A, Tian S, Wang J, Xiao Y, Xu X, Zhang J, Zhao P, Zok T, Westhof E., “RNA-Puzzles Round II: assessment of RNA structure prediction programs applied to three large RNA structures”, RNA, 21(6):1066-84. (2015) [download]
79. DeFever R., Geitner N., Bhattacharya P., Ding F., Ke P.C., Sarupria S., “PAMAM dendrimers and graphene: Materials for removing aromatic contaminants from water”, Environmental Science & Technology, 49:4490-7 (2015) [download]
78. Nedumpully-Govindan P. and Ding F., “Inhibition of IAPP aggregation by insulin depends on the insulin oligomeric state regulated by zinc ion concentration”, Scientific Reports 5, (2015) [download]
77. Wang B., Seabrook S.A., Nedumpully-Govindan P., Chen P., Yin H., Waddington L., Epa V.C., Winkler D.A., Kirby J.K., Ding F., Ke P.C., “Thermostability and Reversibility of Silver Nanoparticle-Protein Binding”, Physical Chemistry Chemical Physics, 17:1728-1739 (2015) [download]
76. Geitner N., Wang B., Andorfer R.; Ladner D.; Ke P.K.; Ding F., “The structure-function relationship of PAMAM dendrimers as robust oil dispersants”, Environmental Science & Technology, 48(21):12868-75, (2014) [download]
75. Homan, P., Tandon, A., Rice, G. M., Ding, F., Dokholyan, N. V., and Weeks, K. M. “RNA tertiary structure analysis and refinement by 2′-hydroxyl molecular interference”, Biochemistry, 53(43):6825-33 (2014) [download]
74. S. Radic, P. Nedumpully-Govindan,R. Chen, E. Salonen, J.M. Brown, P.C. Ke, and F. Ding, “Effect of Fullerenol Surface Chemistry on Nanoparticle Binding-induced Protein Misfolding”, Nanoscale, 6 (14), 8340 – 8349 (2014) [download]
73. P. Nedumpully-Govindan, L. Li, E.G. Alexov, M.A. Blenner, and F. Ding, “Structural and energetic determinants of tyrosylprotein sulfotransferase sulfation specificity”, Bioinformatics, 30(16):2302-9 (2014) [download]
72. P. Toran, I. Smolina, H. Driscoll,F. Ding, Y.J. Sun, C.R. Cantor, and N. Broude, “Labeling native bacterial RNA in live cells”, Cell Research, 24(7):894-7 (2014) [download]
71. Redler, R.L., Shirvanyants, D., Dagliyan, O., Ding, F., Kim, D.N., Kota, P., Proctor, E.A., Ramachandran, S., Tandon, A., and Dokholyan, N.V. “Computational approaches to understanding protein aggregation in neurodegeneration.”, Journal of Molecular Cell Biology, 6(2):104-15 (2014) [download]
70. Y. Wen, N.K. Geitner, R. Chen, F. Ding, P. Chen, R.E. Andorfer, P.N. Govindan, and P.C. Ke, Binding of Cytoskeletal Proteins with Silver Nanoparticles, RSC Advances, 3: 22002-22007, (2013)[download]
69. A. Kakinen, F. Ding, P. Chen, M. Mortimer, A. Kahru, and P.C. Ke, “Interaction of Silver Nanoparticles and Firefly Luciferase and Its Impact on Enzyme Activity”, Nanotechnology, 24: 345101 (2013)[download]
68. F. Ding*, S. Radic, N. Geitner, R. Chen, P. Chen, J.M. Brown and P.C. Ke*, “Direct observation of silver nanoparticle-ubiquitin corona formation”, Nanoscale, online 16 Jul 2013 (2013)[download]
67. S. Radic, N. Geitner, R. Podila, A. Kakinen, P. Chen, P.C. Ke, and F. Ding, “Competitive Binding of Natural Amphiphiles with Graphene Derivatives”, Scientific Reports, on line 24 July (2013) [download]
66. Fourche, D., Muratov, E., Ding, F., Dokholyan, N. V., Tropsha, A. “Predicting binding affinity of CSAR ligands using both structure-based and ligand-based approaches”, Journal of Chemical Information and Modeling, 53: 1915–1922 (2013)[download]
65. N. E. Hudson, F. Ding, I. Bucay, E. T. O’Brien III, O. V. Gorkun, R. Superfine, S. T. Lord, N. V. Dokholyan, and M. R. Falvo, “Submillisecond Elastic Recoil Reveals Molecular Origins of Fibrin Fiber Mechanics” Biophysical Journal, 104:2671–2680 (2013)[download]
64. Dagliyan, O., D. Shirvanyants, A. Karginov, F. Ding, L. Fee, S.N. Chandrasekaran, C.M Freisinger, G. Smolen, A. Huttenlocher, K. M. Hahn, & N. V. Dokholyan, “Rational design of a ligand-controlled protein conformational switch”, Proceedings of the National Academy of Sciences USA, 119(17):6800-6804 (2013)[download]
63. Ramachandran, S.*, Ding, F.*, Weeks, K., and Dokholyan, N. V. “Statistical analysis of SHAPE-directed RNA secondary structure modeling”, Biochemistry, 52:596-599 (2013)[download]
62. F. Ding and N.V. Dokholyan, “Incorporating backbone flexibility in MedusaDock improves ligand binding pose prediction in the CSAR2011 docking benchmark”, J. Chem. Inf. Model., 53:1871-1879 (2013) [download]
61. D.I. Cole, J.D. Legassie, L.N. Bonifacio, V.G. Sekaran, F. Ding, N.V. Dokholyan & M. B. Jarstfer, “New Models of Tetrahymena Telomerase RNA from Experimen- tally Derived Constraints and Modeling”, JACS, 34: 20070–20080 (2012)[download]
60. F. Ding. and Dokholyan, N. V., “RNA three-dimensional structure determination using experimental constraints”, in “RNA Nanotechnology and Therapeutics” edited by Dr. Peixuan Guo, pp 159-176, (2012)[download]
59. Sparta, M., Shirvanyants, D., F., Ding, Dokholyan, N. V., and Alexandrova, A. N. “Hybrid dynamics simulation engine for metalloproteins”, Biophysical Journal, 103:767-776 (2012)[download]
58. F. Ding, C. Lavendar, K.M. Weeks, and N.V. Dokholyan, “Three-Dimensional RNA Structure Refinement by Hydroxyl Radical Probing”, Nature Methods, 9(6):603-608 (2012)[download]
*Accompanied by a news and review paper: Behrouzi, R. and Woodson, S.R., “Rendering RNA in 3D”, Nature Methods, 9(6):552-3 (2012)[link]
57. F. Ding, Y. Furukawa, N. Nukina, and Dokholyan, N.V., “Local unfolding of Cu, Zn superoxide Dismutase monomer determines the morphology of fibrillar aggregates”, Journal of Molecular Biology, 421:548-560 (2012)[download]
56. F. Ding, and Dokholyan, N.V. “Discrete molecular dynamics simulation of biomolecules.” in “Computational Modeling of Biological Systems: From Molecules to Pathways”, E. Springer, (2012) [download]
55. F. Ding and Dokholyan N.V., “Multiscale modeling of RNA Structure and Dynamics” in “RNA 3D Structure Analysis and Prediction”, Edited by Leontis, N. and Westhof, E. Springer, 2012 [download]
54. Nakayama, T., Butler, J. S., Sehgal, A., Severgnini, M., Racie, T., Sharman J., F. Ding, Morskaya, S. S., Brodsky, J., Tchangov, L., Kosovrasti, V., Meys, M., Nechev, L., Wang, G., Peng, C. G., Fang, Y., Maier, M., Rajeev, K. G., Li, R., Hettinger, J., Barros, S., Clausen, V., Zhang, X., Wang, Q., Hutabarat, R., Dokholyan, N. V., Wolfrum, C., Manoharan, M., Kotelianski, V., Stoffel, M. and Sah, D. W. Y. “Harnessing a Physiologic Mechanism for siRNA Delivery with Mimetic Lipoprotein Particles”, Molecular Therapy, 20:1582-9, (2012) [download](2012)
53. Community/Group Publication — Cruz, J. A., Blanchet, M-F., Boniecki, M., Bujnicki, J. M., Chen, S-J., Cao, S., Das, R., F. Ding, Dokholyan, N. V., Flores, S. C., Lavender C. A., Lisi, V., Major, F., Mikolajczak, K., Philips, A., Puton, T., Santalucia, J., Siyenji, F., Hermann, T., Rother, K., Rother, M., Serganov, S., Skorupski, M., Soltysinski, T., Sripakdeevong, P., Tuszynska, I., Weeks, K. M., Waldsich, C., Wildauer, M., Leontis, N. B. and Westhof, E. “RNA-Puzzles: A CASP-like evaluation of RNA three-dimensional structure prediction”, RNA, 18:610-625 [download] (2012)
52. Shirvanyants, D., F. Ding, Tsao, D., Ramachandran, S. and Dokholyan, N. V. “DMD: an efficient and versatile simulation method for fine protein characterization”, Journal of Physical Chemistry B, 116:8375-82 [download] (2012)
51. Dagliyan, O., Proctor, E. A., D’Auria, K., F. Ding* and Dokholyan N. V.* “Structural and Dynamic Determinants of Protein-peptide Recognition”, Structure, 19:1837 (2011) [download]
*Featured in the Journal Cover of Structure.
50. A.W.R. Serohijos, S. Yin, F. Ding, J. Gauthier, D.G. Gibson, V. Setola, W. Maixner, Dokholyan, N.V., and L. Diatchenko, “Structural basis for MOR1 binding and activation”, Structure, 19:1683-1690 (2011) [download]
49. Kota, P.*, F. Ding*, Ramachandran, S.*, and Dokholyan, N.V. “Gaia: automated structure quality assessment of protein models”, Bioinformatics, 27:2209-2215 (2011) [download][service]
48. Proctor, E. A., F. Ding, and Dokholyan, N. V. “Structural and thermodynamic effects of post-translational modifications in mutant and wild type Cu, Zn superoxide dismutase”, Journal of Molecular Biology, 408:555-567 (2011). [download]
47. Ramachandran, S., Kota, P., F. Ding and Dokholyan, N. V. “Automated Minimization of Steric Clashes in Protein Structures”, Proteins: Structure, Function and Bioinformatics, 79:261-270 (2011)[download][service]
46. F. Ding, Yin, S., and Dokholyan, N. V. “Rapid flexible docking using a stochastic rotamer library of ligands”, Journal of Chemical Information and Modeling, 50:1623-32 (2010) [download]
45. A. Karginov, F. Ding, P. Kota, Dokholyan, N.V. and K. Hahn, “Engineered allosteric regulation of kinases in living cells”, Nature Biotechnology, 28:743-748, (2010) [download]
44. C. Lavender, F. Ding, Dokholyan, N.V., K.M. Weeks, “Robust and Generic RNA Modeling Using Inferred Constraints: A Structure for the Hepatitis C Virus IRES Pseudoknot Domain, Biochemistry, 49:4931-4933 (2010)[download]
43. E.A. Proctor, F. Ding, and Dokholyan, N.V., “Discrete Molecular Dynamics”, Wiley Interdisciplinary Reviews: Computational Molecular Science, 1:80-92 (2010)[download]
42. V. V. Lakhani, F. Ding and N. V. Dokholyan, “Poly-glutamine induced misfolding of huntingtin exon1 is modulated by the flanking sequences” Public Library of Science Computational Biology:e1000772 (2010)[download]
41. C. Hajdin, F. Ding, N. V. Dokholyan, and K. M. Weeks, “How good is that RNA tertiary structure prediction?” RNA, 16:1340-1349 (2010) [download] [service]
40. B. A. Kesner, F. Ding, B. S. Temple, and N. V. Dokholyan, “N-terminal strands of filamin Ig domains act as a conformational switch under biological forces” Proteins: Structure, Function, and Bioinformatics, 78:12-24 (2009)[download]
39. M. P. Torres, M. J. Lee, F. Ding, C. Purbeck, B. Kuhlman, N. V. Dokholyan, and H. G. Dohlman, “G Protein Mono-Ubiquitination By the RSP5 Ubiquitin Ligase”, Journal of Biological Chemistry, 284: 8940-8950 (2009)[download]
38. Yin, S., F. Ding and Dokholyan, N. V. “Modeling mutations in proteins using Medusa and discrete molecular dynamics” in “Protein Structure Prediction: Method and Algorithms”, Editors: Rangwala, H. and Karypis, G. Wiley & Sons, (2009)
37. S. Yin, F. Ding, and N. V. Dokholyan, “Computational evaluation of protein stability change upon mutations using Eris.” in “In Vitro Mutagenesis Protocols” Editor: J. Braman. Humana Press (2009)
36. C. M. Gherghe, C. W. Leonard, F. Ding, N. V. Dokholyan, and K. M. Weeks, “Native-like RNA tertiary structures using a sequence-encoded cleavage agent and refinement by discrete molecular dynamics” Journal of the American Chemical Society , 131:2541-2546(2009) [download]
35. F. Ding and N. V. Dokholyan, “Dynamical roles of metal ions and the disulfide bond in Cu, Zn superoxide dismutase folding and aggregation” Proceedings of the National Academy of Sciences USA, 105:19696-19701 (2008) [download]
34. S. Sharma, F. Ding, and N. V. Dokholyan, “iFoldRNA: Three-dimensional RNA structure prediction and folding” Bioinformatics, 24:1951-1952 (2008) [download] [service]
33. D. G. Teotico, M. Frazier, F. Ding, N. V. Dokholyan, B. Temple, and M. R. Redinbo, “Active nuclear receptors exhibit highly correlated AF-2 domain motions” Public Library of Science Computational Biology, 4: e1000111 (2008) [download]
32. F. Ding, D. Tsao, H. Nie, and N. V. Dokholyan, “Ab initio folding of proteins using all-atom discrete molecular dynamics” Structure, 16: 1010-1018 (2008) [download]
31. F. Ding, S. Sharma, P. Chalasani, V. V. Demidov, N. E. Broude, and N. V. Dokholyan, “Large scale simulations of 3D RNA folding by discrete molecular dynamics: From structure prediction to folding mechanisms” RNA, 14: (2008) [download]
30. S. Sharma, F. Ding, and N. V. Dokholyan, “Probing protein aggregation using simplified models and discrete molecular dynamics” Frontiers in Bioscience, 13: 4795-4808 (2008) [download]
29. S. Yin, F. Ding, and N. V. Dokholyan, “Modeling backbone flexibility improves protein stability estimation”, Structure, 15: 1567-1576 (2007) [download]
28. A. R. Lam, J. M. Borreguero, F. Ding, N. V. Dokholyan, S. V. Buldyrev, E. I. Shakhnovich, H. E. Stanley, “Parallel folding pathways in the Src SH3 domain” Journal of Molecular Biology, 373: 1348-1360 (2007) [download]
27. S. Barton, R. Jacak, S. D. Khare, F. Ding*, and N. V. Dokholyan*, “The length dependence of the polyQ-mediated protein aggregation” Journal of Biological Chemistry, 282: 25487-25492 (2007) [download]
26. Y. Chen, F. Ding, H. Nie, A. W. Serohijos, S. Sharma, K. C. Wilcox, S. Yin, and N. V. Dokholyan, “Protein folding: then and now” Archives of Biochemistry and Biophysics, 469: 4-19 (2008) [download]
25. S. Yin, F. Ding, and N. V. Dokholyan, “Eris: An automated estimator of protein stability” Nature Methods, 4: 466-467 (2007) [download][service]
24. Y. Chen, F. Ding, and N. V. Dokholyan, “Fidelity of protein structure reconstruction from inter-residue proximity constraints” Journal of Physical Chemistry B, 111: 7432-7438 (2007) [download]
23. S. Sharma, F. Ding, and N. V. Dokholyan, “Multi-scale modeling of nucleosome dynamics” Biophysical Journal, 92 1457-1470 (2007) [download]
22. A. W. Serohijos, Y. Chen, F. Ding, T. C. Elston, and N. V. Dokholyan, “A new structural model reveals energy transduction in dynein”, Proceedings of the National Academy of Sciences USA, 103: 18540-18545 (2006)[download]
21. S. Sharma, F. Ding, H. Nie, D. Watson, A. Unnithan, J. Lopp, D. Pozefsky, and N. V. Dokholyan, “iFold: A platform for interactive folding simulations of proteins” Bioinformatics, 22: 2693-2694 (2006) [download][service]
20. F. Ding and N. V. Dokholyan, “Emergence of protein fold families through rational design” Public Library of Science Computational Biology, 2: e85 (2006) [download]
19. V. V. Demidov, N. V. Dokholyan, C. Witte-Hoffman, P. Chalasani, H.-W. Yiu, F. Ding, Y. Yu, C. R. Cantor, N. E. Broude, “Fast complementation of split fluorescent protein triggered by DNA hybridization”, Proceedings of the National Academy of Sciences USA, 103: 2052-2056 (2006). [download]
18. F. Ding, J. J. LaRocque, and N. V. Dokholyan, “Direct observation of protein folding, aggregation and a prion-like conformational transition”, Journal of Biological Chemistry, 280: 40235-40240 (2005) [download]
17. F. Ding, K. C. Prutzman, S. L. Campbell, and N. V. Dokholyan, “Topological determinants of protein domain swapping”, Structure, 14: 5-14 (2005). [download][service]
16. S. D. Khare, F. Ding, K. N. Gwanmesia, and N. V. Dokholyan, “Molecular origin of polyglutamine-mediated protein aggregation in neurodegenerative diseases”, PLoS Computational Biology, 1, e30 (2005). [download]
15. F. Ding, W. Guo, N. V. Dokholyan, E. I. Shakhnovich, and J.-E. Shea, “Reconstruction of the src-SH3 protein domain transition state ensemble using multiscale molecular dynamics simulations”, J. Mol. Biol., 350: 1035-1050 (2005). [download]
14. F. Ding and N. V. Dokholyan, “Simple but predictive protein models”, Trends in Biotechnology, 23: 450-455 (2005). [download]
13. F. Ding, R. K. Jha, and N. V. Dokholyan, “Scaling behavior and structure of denatured proteins”, Structure, 13: 1047-1054 (2005). [download]
-Featured as Journal Front Cover
12. F. Ding, S. V. Buldyrev, and N. V. Dokholyan, “Folding Trp-cage to NMR resolution native structure using a coarse-grained protein model”, Biophys. J., 88: 147-155 (2005). [download]
11. R. D. S. Dixon, Y. Chen, F. Ding, S. D. Khare, K. C. Prutzman, M. D. Schaller, S. L. Campbell, and N. V. Dokholyan, “New insights into FAK signaling and localization based on detection of a FAT domain folding intermediate” Structure, 12: 2161-2171 (2004). [download]
10. B. Urbanc, L. Cruz, F. Ding, D. Sammond, S. Khare, S. V. Buldyrev, H. E. Stanley, and N. V. Dokholyan, “Molecular dynamics simulation of Amyloid beta dimer formation” Biophys. J., 87: 2310-2321 (2004). [download]
9. J. M. Borreguero, F. Ding, S. V. Buldyrev, H. E. Stanley, and N. V. Dokholyan, “Multiple folding pathways of the SH3 domain.” Biophys. J. 87: 521-533 (2004). [download]
8. S. Peng, F. Ding, B. Urbanc, S. V. Buldyrev, L. Cruz , H. E. Stanley, and N. V. Dokholyan, “Discrete molecular dynamics simulations of peptide aggregation” Phys. Rev. E 69: 041908 (2004) [download]
7. S. D. Khare, F. Ding and N. V. Dokholyan, “Folding of Cu,Zn superoxide dismutase and Familial Amyotrophic Lateral Sclerosis.” J. Mol. Biol, 334, 515-525, 2003 [download]
6. Dokholyan N.V., Borreguero J.M., Buldyrev S.V., F. Ding, Stanley H.E. and Shakhnovich E.I., Identifying the importance of amino acids for protein folding from crystal structures, Methods in Enzymology Vol. 374: pp 618-640 Macromolecular crystallography D. Editors: C. W. Carter Jr. and R. M. Sweet (2003) [download]
5. F. Ding, Borreguero J.M., Buldyrev S.V., Stangley H.E. and Dokholyan N.V., A mechanism for alpha-helix to beta-hairpin transition, Proteins: Structure, Function and Genetics, 53:220-228 (2003) [download]
4. F. Ding, Dokholyan N.V., Buldyrev S.V., Stanley H.E. and Shakhnovich E.I., Molecular dynamics simulation of C-Src SH3 aggregation suggests a generic amyloidogenesis mechanism, J Mol Biol, 324:851-857 2002 [download]
3. Dokholyan N.V., Li L., Ding F., Shakhnovich E.I., Topological determinants of protein folding, Proceedings of the National Academy of Sciences USA,, 99 (13): 8637-8641 JUN 25 2002 [download]
2. F. Ding, Dokholyan V.N., Buldyrev V.S., Stanley H.E. and Shakhnovich E.I. Direct molecular dynamics observation of protein folding transition state ensemble. Biophys J., 86 (6): December 2002 [download]
1. X.Y. Lei, H. Li, F. Ding, W. Zhang, and N.B. Ming, Novel application of a perturbed photonic crystal: High-quality filter. APPL PHYS LETT 71 (20): 2889-2891 NOV 17 1997 [download]
