The structural and energetic determinants of TPST enzyme specificity
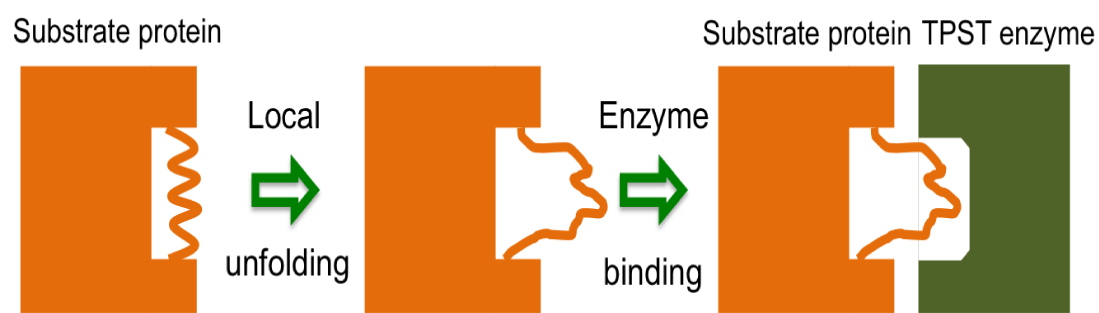
Many PTM enzymes have strong sequence preferences in the targeted substrate proteins/peptides while others do not. What are the molecular mechanism of such drastic differences in PTM enzyme specificities? It is expected that the binding affinity between substrate and enzyme plays an important role in defining the specificities. Interestingly, in the case of tyrosylprotein sulfotransferase proteins (TPST) affinity alone cannot explain the differences in sulfated and non-sulfated sequences. We found that the structural properties of the peptide in the host protein also play an important role in determining the TPST specificity. We are trying to extend this idea to other PTM enzymes.
1. P. Nedumpully-Govindan, L. Li, E.G. Alexov, M.A. Blenner, and F. Ding, “Structural and energetic determinants of tyrosylprotein sulfotransferase sulfation specificity”, Bioinformatics, in press (2014)
